gravimetric method examples|what is gravimetric analysis simple : distributor Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate . web17 de abr. de 2022 · Platense. L W L D L. 16/04/2022 Liga Profesional Argentina Game week 10 KO 19:00. Venue Estadio Pedro Bidegaín (Capital Federal, Ciudad de Buenos .
{plog:ftitle_list}
Resultado da Vestido Paris. A Ghetti Store sempre foi um sonho que começou a se tornar realidade neste ano de 2022 de forma online. Aqui buscamos atender todo .
Types of Gravimetric Methods. The examples in the previous section illustrate four different ways in which a measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call .
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate .
Gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated . Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you .An example of a gravimetric analysis is the determination of chloride in a compound. In order to do a gravimetric analysis, a cation must be found that forms an insoluble compound with chloride. This compound must also be pure . Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of solid.The element to be identified is precipitated from a solution using this method of analysis by .
what is gravimetric analysis simple
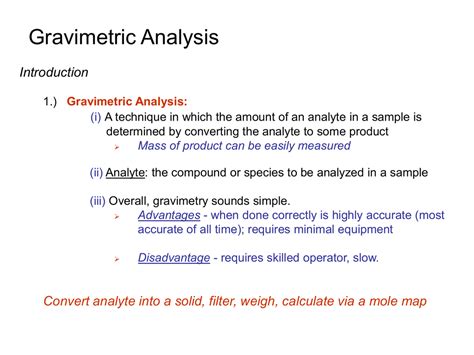
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass.
Gravimetric analysis is a quantitative method in chemistry that involves determining the amount, or concentration, of a substance present in a sample based on the . Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you .
Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction. In a direct precipitation gravimetric analysis, for example, we convert a soluble analyte into an insoluble form that precipitates from solution. In some situations, however, the analyte is .Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass.
steps involved in gravimetric analysis
8A.2 Types of Gravimetric Methods. The four examples in the previous section illustrate different ways in which the measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call the method precipitation gravimetry . The indirect determination of PO. 3 3– by precipitating Hg. 2. Cl. 2. is an ex-
Types of Gravimetric Methods. The examples in the previous section illustrate four different ways in which a measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call the method precipitation gravimetry. The indirect determination of \ . When determining the moisture content in bread, for example, we know that the mass of H 2 O in the bread is the difference between the sample’s final mass and its initial mass. We will return to this concept of applying a conservation of mass later in the chapter when we consider specific examples of gravimetric methods.For example, the moisture (water) content of a sample is routinely determined by measuring the mass of a sample before and after it is subjected to a controlled heating process that evaporates the water. Also common are gravimetric techniques in which the analyte is subjected to a precipitation reaction of the sort described earlier in this .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
steps for gravimetric analysis
An example of a gravimetric analysis is the determination of chloride in a compound. In order to do a gravimetric analysis, a cation must be found that forms an insoluble compound with chloride. This compound must also be pure and easily filtered. The solubility rules indicate that Ag +, Pb 2+, and Hg 2 2+ form insoluble chlorides.This is a summary to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. Skip to main content +- +- chrome_reader_mode . then a particulate gravimetric analysis may be feasible. Examples include the determination of dissolved solids and the determination of fat in foods. 8.5.1 Key Terms.
The publication in 1540 of Vannoccio Biringuccio’s Pirotechnia is an early example of applying gravimetry—although not yet known by this name—to the analysis of metals and ores. 1 Although gravimetry no longer is the most important analytical method, it continues to find use in specialized applications. For example, one standard gravimetric method for the determination of magnesium involves its precipitation as MgNH 4 PO 4 •6H 2 O. Unfortunately, this precipitate is difficult to dry at lower temperatures without losing an .
Gravimetric analysis is one of the commonly used quantitative methods in analytical chemistry, the other being the volumetric method. In gravimetric analysis, the amount of an ion present in an analyte is estimated based on the parameter of mass.
Gravimetric methods were the first techniques used for quantitative chemical analysis, and they remain important tools in the modern chemistry laboratory. The required change of state in a gravimetric analysis may be achieved by various physical and chemical processes. For example, the moisture (water) content of a sample is routinely .An example of a gravimetric analysis is the determination of chloride in a compound. In order to do a gravimetric analysis, a cation must be found that forms an insoluble compound with chloride. This compound must also be pure and easily filtered. The solubility rules indicate that Ag +, Pb 2+, and Hg 2 2+ form insoluble chlorides.Gravimetric analysis is a method of a quantitative assessment of laboratory techniques based mostly on the dimension of an analyte's mass. One example of a gravimetric evaluation technique may be used to decide the quantity of an ion in an answer by way of dissolving a regarded quantity of a compound containing the ion in a solvent to break up the ion from its .The gravimetric method, or the gravimetry, is a technique to determine the hydrogen sorption isotherm at equilibrium state via mass measurement [18].Basically in gravimetry, the pressure is changed in steps, and the hydrogen sorption can be characterized by measuring the mass change of a sample during hydrogenation.
Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . For example, to determine the sulphate ions (SO 4 2-) . Electro gravimetric .GRAVIMETRIC METHOD Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Gravimetry = analytical methods that measure the mass or mass changes. A general principle of gravimetric method of analysis is based on a chemical reaction between analyte and reagent. The analyte (A) of molecules ‘a’ react with the reagent (R) of molecule ‘r’. . For example, the precipitation of calcium oxalate and ignition of it into CaO can be used to determine calcium using gravimetry.Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed .
how to perform gravimetric analysis

Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction.Topic Outcomes • Explain and differentiate the theory and practice of precipitate, volatilization and particulate gravimetry • Determine the quantitative, qualitative application of gravimetric method Overview of Gravimetric • Encompasses all technique in which the signal is a mass or change in mass • Measuring mass is the most .
Gravimetric analysis and volumetric analysis are two common methods used in analytical chemistry to determine the concentration or amount of a substance in a sample. Gravimetric analysis involves the measurement of mass, where the analyte is precipitated and then weighed to determine its concentration.
In most methods the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction generating a precipitate can potentially serve as a gravimetric method. Most precipitation gravimetric methods were developed in the nineteenth century, or earlier, often for the analysis of ores. Figure . One example of a gravimetric analysis technique can be used to determine the amount of an ion in a solution by dissolving a known amount of a compound containing the ion in a solvent to separate the ion from its compound. The ion is then precipitated or evaporated out of solution and weighed. This form of gravimetric analysis is called precipitation gravimetry. Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction.
8A.2 Types of Gravimetric Methods The four examples in the previous section illustrate different ways in which the measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call the method precipitation gravimetry. The indirect determination of PO 3 3– by precipitating Hg 2 Cl 2 is an ex-
how to calculate gravimetric factor
gravimetric factor sample problems
10 de ago. de 2023 · 1 Getting to Wyylde with your smartphone: Android and iOS (Apple) 2 Wyylde doesn't have an app, but its mobile version is highly optimized. 3 Wyylde .
gravimetric method examples|what is gravimetric analysis simple